[ad_1]
Monday marks four years since the World Health Organization (WHO) declared the coronavirus outbreak a pandemic.
Since the first case emerged in Wuhan, China in 2019, millions of people have been infected and died around the world.
There have also been major successes, including vaccines for almost all age groups, antiviral drugs to treat people at risk of severe disease, and widespread use of home tests.
ABC News looks back at some of the biggest moments over the past four years.
December 31, 2019
The World Health Organization’s China office was alerted to a mysterious pneumonia-like illness that originated and is spreading in Wuhan.
January 7, 2020
Chinese public health authorities have identified a new coronavirus as the source of the outbreak.

In this Jan. 17, 2020 file photo, medical staff treat a patient in Wuhan, Hubei province, China, after the city’s second infected person died from the pneumonia-like virus since the outbreak began in December. He was transported to Jinyintan Hospital.
Getty Images, File
January 10, 2020
WHO started using 2019-nCoV to refer to the epidemic. The disease is called SARS-CoV-2 because it resembles the SARS virus.
January 20, 2020
The United States has confirmed its first case, a man in his 30s from Washington state who developed symptoms after traveling to Wuhan.
January 30, 2020
The WHO has declared the virus outbreak a public health emergency of international concern.Centers for Disease Control and Prevention (CDC) confirms human-to-human transmission in the United States
February 11, 2020
The WHO has proposed calling the disease caused by this virus “COVID-19,” short for “coronavirus disease 2019.”
March 11, 2020
WHO has classified the new coronavirus infection as a pandemic.
March 13, 2020
President Donald Trump has declared the coronavirus outbreak a national emergency.
March 19, 2020
The U.S. Department of State has issued a Level 4 “Do Not Travel” health advisory worldwide.
April 2, 2020
More than 1 million people around the world have been confirmed to have been infected with the new coronavirus.
April 3, 2020
The CDC has released new guidelines on mask-wearing, recommending that all Americans wear a mask when outside their homes.
May 15, 2020
President Trump announced Operation Warp Speed, a national plan to speed the development, manufacturing, and distribution of tests, treatments, and vaccines for the novel coronavirus disease (COVID-19).
May 28, 2020
In the United States, more than 100,000 people have died from the coronavirus, just over four months after the first case was confirmed.
August 6, 2020
The State Department has lifted the global Level 4 “Do Not Travel” health advisory.

In this March 11, 2020 file photo, World Health Organization Director-General Tedros Adhanom Ghebreyesus speaks at a press conference in Geneva, Switzerland, calling the coronavirus outbreak a “pandemic” as the virus continues to spread around the world. It has been announced that it can be characterized as .
Xinhua News Agency, via Getty Images, File
August 28, 2020
The first case of reinfection with coronavirus in the United States has been reported by the Nevada State Public Health Laboratory.
September 28, 2020
The number of deaths worldwide due to the new coronavirus infection has exceeded 1 million.
December 11, 2020
The FDA has granted Pfizer-BioNTech its first EUA for a COVID-19 vaccine for people 16 and older.
December 14, 2020
Nurse Sandra Lindsay was the first person to receive the COVID-19 vaccine, which has begun being distributed in the United States.
December 18, 2020
The FDA has granted a similar EUA to Moderna’s vaccine for people 18 and older.
January 12, 2021
The CDC says all airline passengers entering the United States must present a negative coronavirus test result.

In this Dec. 14, 2020 file photo, Sandra Lindsey, a nurse at Long Island Jewish Medical Center, receives the coronavirus vaccine by Dr. Michelle Chester in Queens, New York.
Pool, file from Getty Images
February 27, 2021
FDA issues EUA to Johnson & Johnson’s COVID-19 vaccine for ages 18 and older.
May 10, 2021
The FDA has extended the EUA of the Pfizer-BioNTech COVID-19 vaccine to youth ages 12 to 15.
May 13, 2021
CDC guidance has been updated for fully vaccinated people, eliminating the requirement to wear masks indoors.
May 26, 2021
President Joe Biden has issued a statement saying that U.S. intelligence agencies cannot determine whether the coronavirus disease originated in animals and jumped to humans or whether it arose from a laboratory accident, and there are people who believe the former. There are some who believe in the latter, and others who believe in the latter.
August 12, 2021
The FDA amended the EUAs for Pfizer-BioNTech and Moderna’s COVID-19 vaccines to authorize additional boosters for certain immunocompromised groups.
September 22, 2021
The FDA has authorized additional doses of the Pfizer-BioNTech COVID-19 vaccine for certain groups.
October 6, 2021
WHO has published a definition of a “post-COVID-19 condition” or a clinical case of prolonged COVID-19 infection.
October 20, 2021
FDA approves additional doses of Moderna and J&J COVID-19 vaccines for specific groups.
October 29, 2021
The FDA has authorized emergency use of the Pfizer-BioNTech coronavirus vaccine for children ages 5 to 11.
November 8, 2021The White House announced that all noncitizens traveling to the United States must now be fully vaccinated and provide proof of vaccination status when traveling to the United States. All travelers will still be required to show a negative pre-departure COVID-19 test taken no more than three days before boarding their flight.
November 19, 2021
The CDC has updated its guidance and recommends that everyone age 18 and older receive the COVID-19 booster vaccination.
December 9, 2021
The FDA has expanded eligibility for 16- and 17-year-olds to receive Pfizer-BioNTech’s COVID-19 booster therapy.
December 22-23, 2021
The FDA is introducing two oral antiviral drugs to treat COVID-19 (Merck & Co.’s molnupiravir and Pfizer’s paxlobid) for patients with mild to moderate disease who are at high risk for severe disease. ) issued an EUA.
January 3, 2022
FDA expands the EUA for Pfizer-BioNTech’s booster shot to include people ages 12 to 15 and a third initial dose to some immunocompromised children ages 5 to 11. .
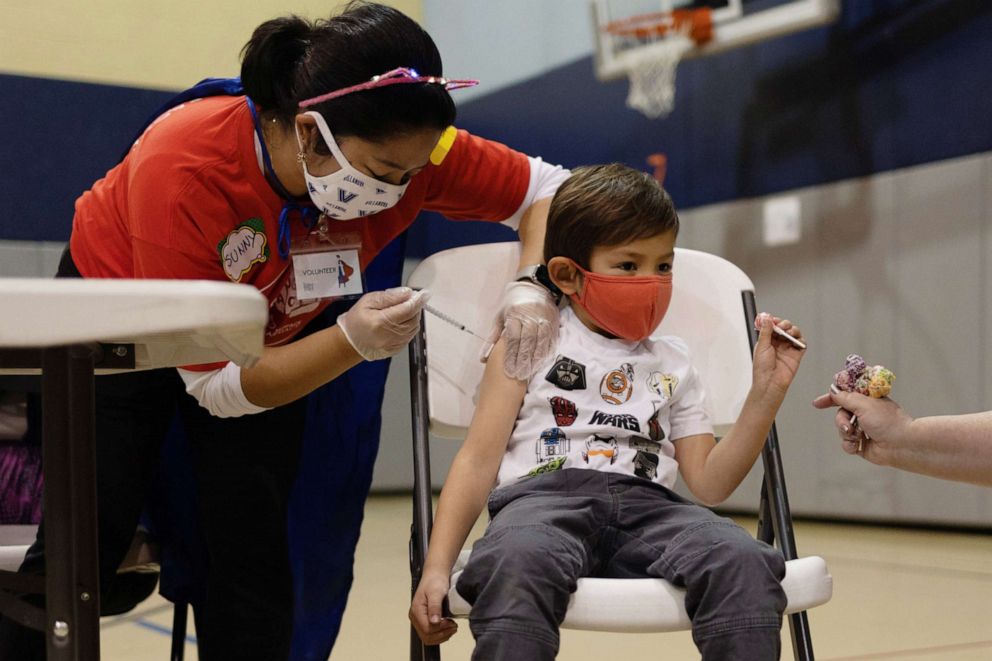
In this Dec. 5, 2021 file photo, a 5-year-old boy is shown receiving the Pfizer-BioNTech COVID-19 vaccine in Lansdale, Pennsylvania.
Hannah Baier/Reuters, File
January 4, 2022
The United States reported more than 1 million new coronavirus infections each day on its first day amid the Omicron wave.
January 15, 2022
In the United States, weekly new hospitalizations due to COVID-19 reached a peak of 150,650 during the Omicron wave.
January 18, 2022The White House has launched a program to mail at-home COVID-19 tests directly to Americans’ homes using a new website.
March 8, 2022
The Biden administration is introducing a “cure for treatment” program that will allow people at high risk of developing severe COVID-19 infections and complications to get tested at a pharmacy and receive free antiviral drugs on the spot if they test positive. announced the start of an inspection.
March 29, 2022
The FDA has authorized second booster doses of the Pfizer-BioNTech or Moderna COVID-19 vaccines for people 60 and older and certain immunocompromised groups.
May 12, 2022The number of deaths due to the new coronavirus infection in the United States has exceeded 1 million. President Biden ordered the flag to be flown at half-staff.
May 17, 2022The FDA has expanded eligibility for a booster dose of the Pfizer-BioNTech COVID-19 vaccine to children ages 5 to 11.
June 10, 2022
The CDC is revoking its order requiring negative coronavirus test results before boarding flights to the U.S., effective June 12.
June 17, 2022
The FDA has approved Pfizer-BioNTech’s COVID-19 vaccine for children 6 months to 5 years of age and Moderna’s COVID-19 vaccine for children 6 months to 6 years of age. The vaccine was granted an EUA.
August 31, 2022
The FDA has authorized the Pfizer-BioNTech and Moderna bivalent COVID-19 vaccines for use as booster shots for people 12 and older and 17 and older, respectively.
October 12, 2022
FDA approves updated bivalent coronavirus disease (COVID-19) vaccines for Pfizer-BioNTech vaccine for children 5 years of age and older and Moderna vaccine for children 6 years of age and older are doing.
December 8, 2022
The FDA has authorized the use of bivalent vaccines as booster shots for children under 5 years of age.
April 10, 2023
President Biden signed HJRes.7, ending the national state of emergency related to the COVID-19 pandemic.
May 5, 2023
The WHO has downgraded the coronavirus disease from a public health emergency of international concern, but still classifies it as a pandemic. The CDC said it will no longer track infection levels.

This undated file photo shows a home self-test kit for coronavirus disease (COVID-19).
UIG, Getty Images, File
September 11, 2023
The FDA has authorized and approved the latest coronavirus vaccine for all Americans six months of age and older.
May 11, 2023
The public health emergency designation for the novel coronavirus disease (COVID-19) in the United States is set to expire.
November 10, 2023
The WHO has updated its coronavirus treatment guidance, recommending the use of antiviral drugs remdesivir and molnupiravir only in severe cases.
March 1, 2024
People recovering from COVID-19 no longer need to quarantine for five days after developing symptoms, according to new guidance from the CDC.
March 10, 2024The Johns Hopkins University Coronavirus Resource Center has stopped collecting data for its popular COVID-19 dashboard.
[ad_2]
Source link


